"The hole in the ozone layer" sounds like a title of a "B
Grade" horror movie, conjuring up all 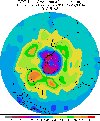 sorts
of misconceptions. It is not a hole in the atmosphere, but a depletion
of ozone above the polar regions which occurs in the spring and summer
months.
sorts
of misconceptions. It is not a hole in the atmosphere, but a depletion
of ozone above the polar regions which occurs in the spring and summer
months.
Not to be confused with "The greenhouse effect", ozone depletion seems to be a relatively new phenomenon, with scientists first suspecting there was a problem in the late 1970s. It was not until 1985 that British scientists discovered the first "hole" over Antarctica.
The hole in the ozone layer A: What is it? investigates how the "hole" forms and what is being done to halt its growth. The second part of this topic will look at the players in the formation of the "hole"; ozone, chlorofluorocarbons and ultraviolet light. T
Ozone
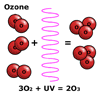 Ozone,
O3, is a form of oxygen gas, which is produced when ultraviolet
light from the Sun strikes and splits an oxygen molecule, O2,
to form two oxygen atoms. These atoms then combine with other oxygen molecules
to form ozone.
Ozone,
O3, is a form of oxygen gas, which is produced when ultraviolet
light from the Sun strikes and splits an oxygen molecule, O2,
to form two oxygen atoms. These atoms then combine with other oxygen molecules
to form ozone.
The high intensity of ultraviolet radiation (UV) from the Sun striking the upper atmosphere ensures that ozone is readily generated in the Earth's upper atmosphere. Unfortunately, ozone is not very stable and can be destroyed by more ultraviolet radiation or contact with other chemicals, especially chlorine atoms.
Ozone can exist fleetingly in the lower atmosphere, soon reacting with other chemicals. Ozone is also created by lightning and electrical discharges and is the odour that can be smelt near arc welding and older photocopying machines.
CFCs: the ozone destroyers
 The
destruction of the ozone in the upper atmosphere is being caused by a
group of chemicals called chlorofluorocarbons or CFCs. CFCs are very stable,
unreactive gases once used as aerosol propellent gases, refrigerants and
in the electronic industry. These gases were used because they were so
unreactive and safe to use in the home. CFCs are still in use in small
quantities, but their use is slowly being phased out.
The
destruction of the ozone in the upper atmosphere is being caused by a
group of chemicals called chlorofluorocarbons or CFCs. CFCs are very stable,
unreactive gases once used as aerosol propellent gases, refrigerants and
in the electronic industry. These gases were used because they were so
unreactive and safe to use in the home. CFCs are still in use in small
quantities, but their use is slowly being phased out.
Because CFCs do not degrade and break down in the atmosphere, they simply accumulated until they reach the upper atmosphere (this can take years and even decades for some of the heavier CFCs). At this point they turn from "Dr Jekyll" to "Mr Hyde". CFCs break apart when struck by ultraviolet light forming different bits of molecules and chlorine atoms. It is these free chlorine atoms, known as radicals, which are so dangerous to the ozone layer.

|
CFCs |
 Ultraviolet light
Ultraviolet light
The third constituent in the ozone drama is ultraviolet light or UV. This
high-energy electromagnetic radiation from the Sun has the ability to
break molecules apart. It is UV that causes sunburn, skin cancer and blindness
from cataracts on the eye. Astronauts must wear protective space suits
to be able survive the high intensity UV coming from our Sun in outer
space. The ozone layer is the Earth's "space suit" and without
it life on Earth would not exist in its present form.
| Copyright owned by the State of Victoria (Department of Education and Early Childhood Development). Used with Permission. |
|
|||||||||||||||||||||||