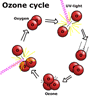
The ozone layer
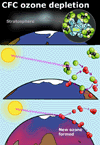
Ozone depletion
Ozone depletion occurs predominantly in the polar regions and mainly over
the South Pole, not over the highly populated regions where the chlorofluorocarbons
CFCs, came from! Depletion occurs in spring and early summer. This depletion
over the polar ice caps in spring and summer is the result of a combination
of factors:
In this way, the ozone is depleted and reformed each year over the poles. The overall quantity of upper atmosphere ozone is slowly declining at about 1% per year. There are signs that we are presently at the worst level of depletion and that steps to stop the use of CFC's are starting to take effect.
The effects of ozone
depletion
 If
ozone depletion were to go on uncontrolled, scientists believe the whole
ozone layer could be at risk. For each 1% decrease in the ozone layer,
ultraviolet light intensity on the surface of the Earth increases by 2%.
Thus, much more ultraviolet light would reach the Earth's surface creating
increased cancer, blindness and mutations in plants and animals. Already,
sheep in the Andes mountains of South America have increased incidence
of blindness.
If
ozone depletion were to go on uncontrolled, scientists believe the whole
ozone layer could be at risk. For each 1% decrease in the ozone layer,
ultraviolet light intensity on the surface of the Earth increases by 2%.
Thus, much more ultraviolet light would reach the Earth's surface creating
increased cancer, blindness and mutations in plants and animals. Already,
sheep in the Andes mountains of South America have increased incidence
of blindness.
Unchecked, life on Earth could be fatally affected, with disease and food shortages dramatically lowering the quality of life and eventually threatening life itself.
What can be done?
Fortunately, a number of protocols have been put in place and the use
of CFCs has been greatly reduced. Currently, scientists hope that the
ozone hole may be already starting to repair itself and ozone depletion
may be a thing of the past by the end of the 21st century.
Unfortunately, no way has been found of removing CFCs from the atmosphere or of generating ozone in the upper atmosphere. Too many CFC chemicals have already been released into the atmosphere to have any chance of removing them now. We will probably just have to ride out the damage to the ozone layer until all the CFCs are naturally removed from the atmosphere and the ozone depletion ceases.
The only thing that can be done is to make sure CFCs are no longer used and released into the atmosphere.
| Copyright owned by the State of Victoria (Department of Education and Early Childhood Development). Used with Permission. |