 There is a basic law in science that energy cannot be created or
destroyed. What happens when a firework explodes in spectacular fashion
in the sky? It seems at first sight that everything disappears, except
for a charred case you might find on the ground. In fact, the energy stored
in the firework has been changed to other forms - it has been transformed.
There is a basic law in science that energy cannot be created or
destroyed. What happens when a firework explodes in spectacular fashion
in the sky? It seems at first sight that everything disappears, except
for a charred case you might find on the ground. In fact, the energy stored
in the firework has been changed to other forms - it has been transformed.
Identifying energy transformations
Let's see if we can identify some of the energies involved in this transformation
sequence. From the moment the fuse is lit, thermal and light energy are
apparent, but this is minor compared with the energy about to be released!
With a whoosh, the whole thing is rocketed into the sky, leaving
a trail of sparks - sound, light and thermal energy. The big explosion
follows, with a thunder and crash of almost deafening sound! Finally,
a cascade of brilliant colours fall towards the ground, with the remnant
of the case.

|
Firework energy |
So far you will have noticed at least four forms of energy: light, sound, thermal and kinetic (energy of movement). Where does all this energy come from? To answer this question, you need to go back to the firework itself. It must have all of this energy stored, ready to be released. It has great potential as you have seen, so this stored energy is called potential energy.
Chemical energy at work
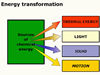 The type of potential energy stored in this case is more commonly
known as chemical energy. It is this stored energy that is finally transformed
into all the other forms of energy that go to make up the dramatic display.
All the materials in the Universe are made up of atoms, ions, molecules
or bits of atoms, most of them are fairly stable but some are readily
able to chemically react, releasing the energy stored in them.
The type of potential energy stored in this case is more commonly
known as chemical energy. It is this stored energy that is finally transformed
into all the other forms of energy that go to make up the dramatic display.
All the materials in the Universe are made up of atoms, ions, molecules
or bits of atoms, most of them are fairly stable but some are readily
able to chemically react, releasing the energy stored in them.
Some of the chemical energy stored in the fireworks is converted to kinetic energy to send the rocket skyward; some compresses the air rapidly, producing sound and still more energy is transformed into light of many colours.
Other chemical transformations
Chemicals like petrol and LPG have lots of stored chemical potential energy
that can propel cars, for example, by being transformed into other forms
of energy. An explosion increases the pressure of gas that pushes pistons
in the car engine (kinetic energy) then, by a series of gears, turns the
car's wheels, and moves the car forward. Again, the chemical energy is
transformed into thermal energy, sound and the kinetic energy of movement.
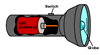 An electric cell (battery) is another type of chemical energy store.
This time, it supplys energy to electrons. This stored electrical energy
can be transformed into sound, thermal, light or kinetic energy depending
on the components in the circuit. For example, a torch transforms electrical
energy to light energy, with some of the energy being "wasted"
as thermal energy - you can observe these effects if you touch a small
torch light globe that has been operating for a while. (Be careful - mains
light globes can get very hot indeed.)
An electric cell (battery) is another type of chemical energy store.
This time, it supplys energy to electrons. This stored electrical energy
can be transformed into sound, thermal, light or kinetic energy depending
on the components in the circuit. For example, a torch transforms electrical
energy to light energy, with some of the energy being "wasted"
as thermal energy - you can observe these effects if you touch a small
torch light globe that has been operating for a while. (Be careful - mains
light globes can get very hot indeed.)

|
Energy transformation in a torch |
| Copyright owned by the State of Victoria (Department of Education and Early Childhood Development). Used with Permission. |