You will have heard of the word light and think about the light you can see from the Sun or the lights in your room or from this computer screen. There are actually many forms of light that we cannot see, but can feel or see the effects of their presence. Light is a form of radiation energy which exists as tiny packets called photons.
The whole electromagnetic spectrum
There is a great range of radiation energy, called electromagnetic radiation,
that travels at the speed of light, which is about 300 000 km/s in space.
This whole range of energy is called the electromagnetic spectrum. Each
section of the spectrum has different energy and different uses, but all
are important in our everyday life.
Wavelength and energy
 Electromagnetic radiation (EMR) is best described in terms of wavelength.
Like the waves coming ashore at a beach, EMR has a distance between waves
known as the wavelength. As wavelength increases, the energy of the photons
making up the EMR decreases. The electromagnetic spectrum ranges from
radiowaves with wavelengths of many kilometres to gamma radiation with
a wavelength measuring very tiny fractions of a millimetre.
Electromagnetic radiation (EMR) is best described in terms of wavelength.
Like the waves coming ashore at a beach, EMR has a distance between waves
known as the wavelength. As wavelength increases, the energy of the photons
making up the EMR decreases. The electromagnetic spectrum ranges from
radiowaves with wavelengths of many kilometres to gamma radiation with
a wavelength measuring very tiny fractions of a millimetre.
Visible
light
 The light you can actually see is called visible light, and it forms
only a very tiny part of the whole spectrum. Its position in the spectrum
is such that it has from 4 × 10-7 metres (the "blue end")
to 7 × 10-7 metres (the "red end").
The light you can actually see is called visible light, and it forms
only a very tiny part of the whole spectrum. Its position in the spectrum
is such that it has from 4 × 10-7 metres (the "blue end")
to 7 × 10-7 metres (the "red end").
Sunburn and the spectrum
Moving just outside the visible light spectrum are infrared (below red)
and ultraviolet (beyond violet).
Infrared rays cause the body to feel warm; for example, infrared heaters radiate energy which then raises the temperature of your skin, creating a feeling of warmth. Infrared has a longer wavelength than the red end of visible light, and lower energy per photon.
 Ultraviolet
radiation has a shorter wavelength than visible and can damage your skin
as it has greater energy per photon. It is this ultraviolet radiation
that sunscreens are designed to filter out.
Ultraviolet
radiation has a shorter wavelength than visible and can damage your skin
as it has greater energy per photon. It is this ultraviolet radiation
that sunscreens are designed to filter out.
Microwaves and radios
At a longer wavelength of the EMR spectrum are microwaves. Microwave ovens
use microwaves to vibrate water molecules in food. By increasing the vibration
energy of molecules of water, the temperature is increased in the food.
This is the way that food in microwave ovens can be heated without having
to raise the temperature of its surroundings.
Note: Microwaves will also excite electrons in metals creating sparks and electrical discharges. This is why metal should never be place in a microwave oven.
Radio waves are at the even longer wavelength end of the EMR spectrum and can carry radio signals great distances, even through buildings. We are not generally aware of their existence and need special receivers, radios tuned to their specific frequency, to detect them.
X-rays and Gamma rays
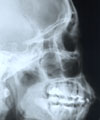 This radiation is at the short wavelength end of the EMR spectrum
and has a very high energy per photon. X-rays can penetrate flesh, but
not bones or teeth, so can be used to "look" inside the body
to observe what is happening below the surface.
This radiation is at the short wavelength end of the EMR spectrum
and has a very high energy per photon. X-rays can penetrate flesh, but
not bones or teeth, so can be used to "look" inside the body
to observe what is happening below the surface.
| Copyright owned by the State of Victoria (Department of Education and Early Childhood Development). Used with Permission. |