When your kettle boils, water evaporates and becomes a gas called water vapour - the steam you see coming from the spout is this vapour condensing again in the cooler air. Water vapour has a property that is so very important in everyday life; it takes up much more volume than the liquid water it came from. The force produced by steam is so powerful that it can drive large steam engines and turn turbines of power stations to generate electricity.
The steam engine
 An Egyptian named Hero developed a steam engine about 120 BC. He
made a small hollow globe with a pipe through the centre connected to
a vessel producing steam. On both sides of the sphere he fixed two L-shaped
tubes so that the steam issued from them and caused the sphere to spin.
It was not able to do any real work but did demonstrate the power of steam.
An Egyptian named Hero developed a steam engine about 120 BC. He
made a small hollow globe with a pipe through the centre connected to
a vessel producing steam. On both sides of the sphere he fixed two L-shaped
tubes so that the steam issued from them and caused the sphere to spin.
It was not able to do any real work but did demonstrate the power of steam.
 James
Watt was said to have thought of the idea of a steam engine by watching
steam issuing from a kettle of boiling water - not likely but a good story!
James
Watt was said to have thought of the idea of a steam engine by watching
steam issuing from a kettle of boiling water - not likely but a good story!
 James Watt actually improved on existing steam engines, taking out
his first patent in 1769. Further improvements by many engineers over
the years culminated in the steam engines that could power locomotives
and the propellers of ocean liners and freighters.
James Watt actually improved on existing steam engines, taking out
his first patent in 1769. Further improvements by many engineers over
the years culminated in the steam engines that could power locomotives
and the propellers of ocean liners and freighters.
Thermal power stations
Nuclear, coal powered, oil and gas burning power stations all work by
heating water under pressure to create high energy steam. This high pressure,
high temperature steam is used to turn turbines which, when attached to
electrical generators, produce useful electrical energy. The vital link
in the transformation of the stored potential energy in the fuel and the
electrical energy produced is the ability of steam to efficiently transfer
thermal energy.
Cooking under pressure
 In a pressure cooker, water is heated and a weight is placed on the
only exit from an otherwise sealed unit. Steam produced in the pressure
cooker forces against the weight and the pressure inside the cooker increases
until the weight is lifted. Depending on the weight used, pressures much
higher than the normal 1 atmosphere pressure can be reached inside the
cooker. Water boils when the vapour pressure exceeds the atmospheric pressure.
By increasing the pressure within the cooker, the energy needed by the
water molecules to
boil has to increase as well. Hence, the water
boils at a higher temperature. The food gets
much hotter by this process and therefore it cooks faster.
In a pressure cooker, water is heated and a weight is placed on the
only exit from an otherwise sealed unit. Steam produced in the pressure
cooker forces against the weight and the pressure inside the cooker increases
until the weight is lifted. Depending on the weight used, pressures much
higher than the normal 1 atmosphere pressure can be reached inside the
cooker. Water boils when the vapour pressure exceeds the atmospheric pressure.
By increasing the pressure within the cooker, the energy needed by the
water molecules to
boil has to increase as well. Hence, the water
boils at a higher temperature. The food gets
much hotter by this process and therefore it cooks faster. 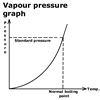
Vapour pressure and boiling
point
There is a simple relationship between vapour pressure and boiling point.
This graph shows how the boiling point varies with vapour pressure. As
the vapour pressure inside the cooker is increased, the temperature at
which the liquid boils also increases.
| Copyright owned by the State of Victoria (Department of Education and Early Childhood Development). Used with Permission. |