All matter (anything made up of atoms) is divided into four physical
"states": solid, liquid, gas and plasma. The chemical composition
of a substance determines its state at any particular temperature. ![]()
All substances start as solids at the very lowest possible temperature, absolute zero (-273°C) and go through changes of state as they are heated. As a solid is heated, it will generally melt to become a liquid, boil to form a gas and finally, at extreme temperatures like those found on the Sun or a very hot flame, its atoms will break apart to become a plasma.
The states of matter on Earth
 Plasma, in the form of ionised gas, is very rare on Earth and usually
only exists fleetingly as the glowing gas in a flame or as a bolt of lightning.
Gases are mainly found in the Earth's atmosphere, although small pockets
of gas are also caught in the Earth's mantle. Most of the outer part of
our planet is solid; this solid mantle sits on an outer liquid core and
a solid inner core. Two thirds of the Earth's surface is covered in liquid
water.
Plasma, in the form of ionised gas, is very rare on Earth and usually
only exists fleetingly as the glowing gas in a flame or as a bolt of lightning.
Gases are mainly found in the Earth's atmosphere, although small pockets
of gas are also caught in the Earth's mantle. Most of the outer part of
our planet is solid; this solid mantle sits on an outer liquid core and
a solid inner core. Two thirds of the Earth's surface is covered in liquid
water.
The states of matter in the Universe
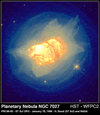 The most common state in the Universe is plasma. This is because
most of the atomic matter in the Universe is found in stars and stars
are massive, extremely hot balls of ionised gas or plasma.
The most common state in the Universe is plasma. This is because
most of the atomic matter in the Universe is found in stars and stars
are massive, extremely hot balls of ionised gas or plasma.
Gas is the next most universally common state. It is found in vast gas nebula clouds spread throughout space and in the giant gas planets like Jupiter and Saturn.
Solid forms the third most common state. It is found in the form of cosmic dust, Earth-like planets, comets and other stray pieces of rock.
Liquid is probably the rarest state in the Universe, with the only discovered naturally occurring liquids being the Earth's surface water and our liquid metal core. Some astronomists believe that there may be water on a few of the moons in the outer Solar System and that water may have once existed on Mars and carved out its extensive canyons.
What makes one state different from
another?
The state of a substance at any particular temperature and pressure is
dependent on how big its particles are and how strongly they bond together.
For example, substances which are solids under normal conditions (25°C
and 1 atmosphere pressure) are usually made of large heavy atoms or molecules
and are tightly bonded together. On the other hand, gases under these
same conditions tend to be made of small, light atoms or very small molecules
that do not stick together well. Liquids generally have particles which
are somewhere in between these two extremes.
 When
substances undergo a change of state they generally do not chemically
change, or form new substances. They only undergo physical changes. Water
is made of H2O molecules, whether it is ice, liquid water or
steam. Changes in state are usually easily reversed, by either heating
or cooling, whereas chemical changes are more difficult to reverse.
When
substances undergo a change of state they generally do not chemically
change, or form new substances. They only undergo physical changes. Water
is made of H2O molecules, whether it is ice, liquid water or
steam. Changes in state are usually easily reversed, by either heating
or cooling, whereas chemical changes are more difficult to reverse.
| Copyright owned by the State of Victoria (Department of Education and Early Childhood Development). Used with Permission. |