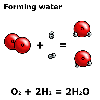It is suggested that the topic Reading
chemical formulas be read before undertaking this topic. Chemists
have a special shorthand way of writing the names of chemicals and how
they behave in chemical reactions.
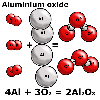 For
example, water has the formula H2O and when aluminium combines
with oxygen in the air to corrode, chemists write the equation:
For
example, water has the formula H2O and when aluminium combines
with oxygen in the air to corrode, chemists write the equation:
- 4 Al + 3 O2 ![]() 2 Al2O3 for the reaction.
2 Al2O3 for the reaction.
This topic will help you to read what chemists mean when they write chemical equations.
Formulas and chemical reactions
Chemists have formulas or shorthand names for all atoms, ions, molecules
and ionic salts known. These formulas list the types of atoms and number
of each atom present in a substance.
During chemical reactions, substances are broken down and new substances formed. Once a chemist has determined which substances are involved in a reaction their formulas can be determined using the rules of valency and covalency. (See the related topics for more information on valency and covalency.) The formula of a substance is set by these rules and is always the same. Water always has the formula H2O and never varies!
Reactants and products
The substances that undergo a chemical reaction are known as "the
reactants" and the new substances produced are called "the products".
In a chemical equation, the reactants are written on the left and the
products on the right with an arrow in the middle denoting a reaction
has taken place.
Reactants ![]() Products
Products
If more than one reactant is involved in the reaction or more than one product produced, then plus signs (+) are used between the formulas of the substances involved.
Reactant 1 + Reactant 2 ![]() Product 1 + Product 2
Product 1 + Product 2
In the initial example of the corrosion of aluminium, aluminium and oxygen
are the reactants and aluminium oxide is the single product.
4 Al + 3 O2 ![]() 2 Al2O3
2 Al2O3
What do the numbers mean?
Remember, the subscript numbers, like the 2 in O2, are part
of the formulas and denote the number of atoms of each type present in
the formulas of the substance. The larger numbers in front of the formulas
are part of the chemical equation and denote the number of units of each
atom or compound reacted or produced as part of the reaction.
 At
a very simple level, the equation 4 Al + 3 O2
At
a very simple level, the equation 4 Al + 3 O2 ![]() 2 Al2O3 can be interputed to mean:
2 Al2O3 can be interputed to mean:
"Four atoms of aluminium and three molecules of oxygen react to form two units of aluminium oxide".
Note: Aluminium oxide exists as cyrstals in a lattice like salt or sand. Strictly speaking, we cannot describe the units of aluminium oxide as molecules.
Atoms are always shown as single elementary formulas with no subscripts. For example: H, He, Al, C and Ca. Molecules are composed of two or more atoms, for example: O2, H2O and C6H12O6.
Note: Like all topics, this subject has been dealt with at a fundamental level and the difference between covalent molecules and ionic salts is not tackled here.
Here are two more equations and their readings:
"Two hydrogen molecules and an oxygen molecule react to form two water molecules".
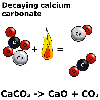 According
to the equation, when lime, calcium carbonate, CaCO3
is heated it breaks down into calcium oxide, CaO, and carbon dioxide,
CO2.
According
to the equation, when lime, calcium carbonate, CaCO3
is heated it breaks down into calcium oxide, CaO, and carbon dioxide,
CO2.
|
heat
|
||
|
CaCO3 |
|
CaO + CO2 |
"A unit of calcium carbonate breaks down on heating to form a unit of calcium oxide and a molecule of carbon dioxide".
Note: Calcium carbonate and calcium oxide exists as cyrstals in a lattice. Strictly speaking, we cannot describe the units of calcium carbonate and calcium oxide as molecules.
The only other feature of an equation that is commonly used is the state
of the substances involved; solid, liquid, gas or water solution.
- Solid - is denoted by a subscript (s)
after the formula of the substance, CaO(s).
- Liquid - is denoted by a subscript (l)
after the formula of the substance, H2O(l).
- Gas - is denoted by a subscript (g) after
the formula of the substance, CO2(g).
- Water solutions are called "aqueous solutions" and are denoted by the subscript (aq) after the formula of the substance, NaCl(aq).
The heating of lime thus becomes:
|
heat
|
||
|
CaCO3(s)
|
|
CaO(s)
+ CO2(g)
|
"A unit of solid calcium carbonate breaks down on heating, to form a unit of solid calcium oxide and a molecule of gaseous carbon dioxide".
The formation of water becomes:
2 H2(g) + O2(g) ![]() 2 H2O(l)
2 H2O(l)
"Two molecules of gaseous hydrogen and a molecule of gaseous oxygen react to form two molecules of liquid water".
| Copyright owned by the State of Victoria (Department of Education and Early Childhood Development). Used with Permission. |