|
NaCl, sodium chloride or common
salt
Common salt (sodium chloride being its scientific name) is the best
known example of the large, important group of compounds known collectively
as "salts". Sodium chloride displays many of the properties
associated with salts and, in many ways, is a typical salt. We will
first investigate the properties of common salt and then expand on this
knowledge to find out more about salts in general in the topic Ions
and salts.
Common salt - sodium chloride
 Salt
is a clear, white, crystalline solid with a high melting point of 801°C.
It shatters when hit with a hammer, forming many smaller crystals. Salt
will not cut like wood or butter, but will cleave along a straight face.
It is quite soluble in water, but will not dissolve in petrol or other
liquid hydrocarbons. Salt
is a clear, white, crystalline solid with a high melting point of 801°C.
It shatters when hit with a hammer, forming many smaller crystals. Salt
will not cut like wood or butter, but will cleave along a straight face.
It is quite soluble in water, but will not dissolve in petrol or other
liquid hydrocarbons.
 Salt
is collected by two common methods: mining "rock salt" from
underground salt deposits, laid down millions of years ago by old oceans
and seas, or by evaporating off the water from sea water leaving behind
"sea salt". Chemically, rock salt and sea salt are the same
- sodium chloride with traces of other minerals and salts. They differ
only in the size of their crystals, with rock salt usually having larger
crystals. Salt
is collected by two common methods: mining "rock salt" from
underground salt deposits, laid down millions of years ago by old oceans
and seas, or by evaporating off the water from sea water leaving behind
"sea salt". Chemically, rock salt and sea salt are the same
- sodium chloride with traces of other minerals and salts. They differ
only in the size of their crystals, with rock salt usually having larger
crystals.
Made of ions
Sodium chloride is made from sodium ions and chloride ions. The formula
for sodium chloride is NaCl. Both sodium and chloride ions are very
common on Earth, being found in the oceans, rocks and soil.
In salt water solution, sodium and chloride ions move about independent
of each other. In solid form however, these two ions form a close one-to-one
relationship.
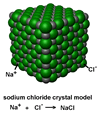 Salt crystals are not the same as molecules where two or more atoms
combine to form single discrete molecules. In an ionic salt crystal
a lattice of sodium and chloride ions is formed which grows into a crystal.
The formula NaCl tells us that the ratio of sodium ions (Na+) to chloride
ions (Cl-) is one-to-one but, unlike in a molecule, the actual number
of ions in a single crystal can be incredibly large.
Salt crystals are not the same as molecules where two or more atoms
combine to form single discrete molecules. In an ionic salt crystal
a lattice of sodium and chloride ions is formed which grows into a crystal.
The formula NaCl tells us that the ratio of sodium ions (Na+) to chloride
ions (Cl-) is one-to-one but, unlike in a molecule, the actual number
of ions in a single crystal can be incredibly large.
|