|
From atoms to molecules
 If
atoms are the basic building blocks of matter, then molecules are the
simplest constructions. Molecules are made up of atoms, not just thrown
together, but placed together using strict rules of numbers and patterns.
Molecules are the smallest discrete units which possess the unique chemical
and physical properties of the substance they make up. If
atoms are the basic building blocks of matter, then molecules are the
simplest constructions. Molecules are made up of atoms, not just thrown
together, but placed together using strict rules of numbers and patterns.
Molecules are the smallest discrete units which possess the unique chemical
and physical properties of the substance they make up.
Making a molecule
 Molecules
are formed when two or more atoms combine to form a new unit of matter.
The properties of the resulting molecule are always vastly different
from the combining atoms. For example, water is formed when hydrogen
and oxygen (two highly reactive gases) combine. In fact single atoms
do not commonly exist in nature. Most molecules are formed when atoms
from other molecules, which have broken apart, recombine in a new way.
This is explained in more detail in the topic Chemical reactions. Molecules
are formed when two or more atoms combine to form a new unit of matter.
The properties of the resulting molecule are always vastly different
from the combining atoms. For example, water is formed when hydrogen
and oxygen (two highly reactive gases) combine. In fact single atoms
do not commonly exist in nature. Most molecules are formed when atoms
from other molecules, which have broken apart, recombine in a new way.
This is explained in more detail in the topic Chemical reactions.
 Water is written by chemists as H2O, this means that
two hydrogen atoms and one oxygen atom have combined to form one water
molecule, H2O.
Water is written by chemists as H2O, this means that
two hydrogen atoms and one oxygen atom have combined to form one water
molecule, H2O.
Molecular formulas
The formula of a molecule is set down by the laws of nature which control
the way atoms combine to form molecules. They cannot be changed without
the substance changing too, most combinations simply will not work and
the molecule simply falls apart if formed. For example, water (H2O)
is one compound but hydrogen peroxide (H2O2),
not much different except for one extra oxygen atom, is another compound.
While water is harmless, hydrogen peroxide is a deadly substance that
will kill you if you drink it or swim in it - it's very different from
water!
Here are some more common molecules and their formulas, see if you
can work out how many atoms of each element are present.
|
elementary molecules
|
compound molecules
|
|
oxygen gas O2
|
propane gas C3H8
|
|
hydrogen gas H2
|
hydrochloric acid HCl
|
|
nitrogen gas N2
|
sulfuric acid H2SO4
|
|
chlorine gas Cl2
|
carbon dioxide CO2
|
Elementary and compound
molecules
You may have noticed the molecules on the left were made up of one type
of atom only. These are still known as elements although they are no
longer single atoms. Those on the right were made of two types of atoms,
thus they are compounds.
All that is needed is two or more atoms to make a molecule.
If all the atoms are the same it is an elementary molecule, if they
are different it is a compound molecule.
Molecules and life
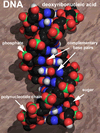 All living things are made of cells and cells are made of molecules.
Some molecules involved in making life possible can be very simple,
like oxygen and water, while other molecules, deoxyribonucleic acid
(DNA), can contain millions of atoms in complex structures with just
as complex names! The following illustration is a representation of
a DNA molecule with its twisted double helix structure.
All living things are made of cells and cells are made of molecules.
Some molecules involved in making life possible can be very simple,
like oxygen and water, while other molecules, deoxyribonucleic acid
(DNA), can contain millions of atoms in complex structures with just
as complex names! The following illustration is a representation of
a DNA molecule with its twisted double helix structure.
|