|
Changes in state - melting and freezing
 Melting
and freezing refer to the changes in state which occur when the solid
and liquid states interchange. Melting occurs when a solid is heated
and turns to a liquid and freezing occurs when a liquid is cooled and
turns to a solid. Melting
and freezing refer to the changes in state which occur when the solid
and liquid states interchange. Melting occurs when a solid is heated
and turns to a liquid and freezing occurs when a liquid is cooled and
turns to a solid.
Melting - solid to liquid
When a solid is heated, its particles gain enough
energy to overcome the bonding forces holding them firmly in place.
They start to move about, but stay close to their neighbouring particles,
rolling around each other. This particle movement allows the substance
to flow and form a liquid. Solids and liquids are similar in their density,
that is, number of particles per unit volume. They differ only in the
ability of the liquid to flow and change shape to occupy the shape of
the bottom part of its container.
Melting
point of pure and impure substances
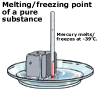 For pure substances, the temperature at which melting and freezing occurs
is quite sharp and is called the melting point of the substance. For
impure substances, melting and freezing occur more gradually over a
range of temperature. This is one way that chemists identify the purity
of a substance; a pure substance will melt at a set temperature while
the more impure a substance is, the more its melting point will vary
over a range of temperatures.
For pure substances, the temperature at which melting and freezing occurs
is quite sharp and is called the melting point of the substance. For
impure substances, melting and freezing occur more gradually over a
range of temperature. This is one way that chemists identify the purity
of a substance; a pure substance will melt at a set temperature while
the more impure a substance is, the more its melting point will vary
over a range of temperatures.
 Freezing
- liquid to solid Freezing
- liquid to solid
Freezing occurs when a liquid is cooled and turns to a solid. Upon cooling,
the particles in a liquid lose energy, stop moving about and settle
into a stable arrangement, forming a solid. Freezing occurs at the same
temperature as melting, hence, the melting point and freezing point
of a substance are the same temperature. The melting/freezing point
of a substance is defined as the temperature above which, the substance
is liquid and below which, it is solid.
Melting points
Different solids have different melting points depending on the strength
of bonding between the particles and the mass of the particles. Essentially,
the heavier the particles in the solid, and the stronger the bonding,
the higher the melting point.
Helium, the lightest and weakest bonded substance known, has a melting
point very near absolute zero, or - 273°C. At the other end of the
scale, Tungsten, made of much heavier particles with very strong bonding,
melts at 3410°C.
|