|
Element, compound or mixture?
Matter is commonly found in three chemical forms - elements, compounds
and mixtures. These are not to be confused by the physical forms of
matter - solids, liquids, gases and plasma. All matter is made of atoms,
but it is how these atoms are combined that determines their status
as an element, compound or mixture.
Elements
Elements are substances made up entirely of one
type of atom only. They can be made of single atoms like helium (He),
molecules like oxygen (O2), or bonded solids like the metals,
diamond and silicon. The Periodic Table lists all the known elements,
and is divided into three groups: metals, metalloids and non-metals.
Compounds
Compounds contain different atoms in set proportions: they have formulas
set down by the laws of nature. For example, water (H2O)
is one compound and hydrogen peroxide (H2O2),
not much different except for one extra oxygen atom, is another compound.
The atoms in a compound are chemically combined in proportions by mass
that are always the same for a particular compound. Compounds are easy
to identify if you have their formula. Only compounds have formulas
with two or more different types of atoms or elements.
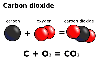 Carbon
dioxide has the formula CO2 therefore it is a compound. Carbon
dioxide has the formula CO2 therefore it is a compound.
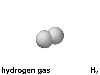 Hydrogen, with the formula H2, is an element, not a compound.
Hydrogen, with the formula H2, is an element, not a compound.
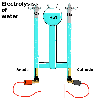 Compounds
can be decomposed into their constituent elements. An example is water
being chemically decomposed into its two elements, hydrogen and oxygen,
by electrolysis: passing electricity though a water solution. Compounds
can be decomposed into their constituent elements. An example is water
being chemically decomposed into its two elements, hydrogen and oxygen,
by electrolysis: passing electricity though a water solution.
Mixtures
Mixtures occur when elements, and/or compounds, are physically mixed
together without their atoms chemically combining. Mixtures are most
common in the natural world. Sea water, air and soil are all mixtures.
Mixtures do not have formulas and their composition is variable. For
example, sea water from the Dead Sea is saltier than water from the
Pacific Ocean and air can be clean or polluted.
Mixtures can be separated into three categories according
to the size of the individual particles:
- Coarse or crystalline mixtures with components that are visible
to the naked eye or microscope - cement, granite rock, wood.
- Colloids and emulsions with component particles too small to see,
but big enough to scatter light - jelly, ink and milk.
- Solutions, mixtures on the atomic and molecular scale, usually transparent
to light, but may be coloured and absorb some colours of visible light
- soda water, glass and air.
All mixtures can be physically separated to obtain their component
elements and compounds. (Sometimes this is not an easy process and requires
considerable laboratory skills). They do not have formulas, but can
have recipes with differing proportions of ingredients!
|