As stated in Atoms, every thing is made of atoms - rocks, living things, the Earth, the stars, all that we can see. Now we will investigate the types of atoms that exist and how we describe them.
Over 100 elements
There are over 100 different known atoms and each of these atoms represents
an element. For example, hydrogen atoms, with their 1 proton and 1 electron,
compose the element hydrogen. The element uranium is made up of atoms
with 92 protons and 92 electrons and an average of 146 neutons.
The elements are the macroscopic manifestations of their atoms. When
scientists talk about the element sodium, they say it is a soft, reactive
metal. When they talk about sodium atoms, they say they have 11 protons,
11 electrons and generally 12 neutrons.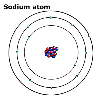
 The
two terms, "elements" and "atoms" are quite often
used in place of each other. Remember, "element" is the correct
word for the substance and "atom" for the individual particles
making up the element.
The
two terms, "elements" and "atoms" are quite often
used in place of each other. Remember, "element" is the correct
word for the substance and "atom" for the individual particles
making up the element.
Atomic numbers
The number of protons in an atom determines its chemical properties and
hence which element the atom belongs to. The atomic number of an atom
is the number of protons it has and is the best way of cataloguing atoms.
For example: all helium atoms have 2 protons and atomic number 2 and
all iron atoms have 26 protons and atomic number 26
Atomic mass
The second important number to know about is an atom's atomic mass. This
is the average mass of an atom of an element. The relative atomic mass
of an element is determined by comparing the average mass of the atoms
of an element on a scale where hydrogen equals approximately 1 (the real
definition is some what more complex as it is now based on Carbon -12
= 12, but the result is the same). On this scale, the average iron atom
has a mass of 55.8; that is, the average iron atom is 55.8 times heavier
than the average hydrogen atom.
The Periodic Table
 All
the known elements can be listed on a special table called the Periodic
Table. The Periodic Table lists all the known elements in order of increasing
atomic number and arranges them into groups (vertical columns) and periods
(horizontal rows) according to how the electrons are arranged in each
atom. It turns out that atoms in the same group have similar arrangements
of electrons and similar chemical and physical properties. This can be
very handy in understanding the properties of the elements.
All
the known elements can be listed on a special table called the Periodic
Table. The Periodic Table lists all the known elements in order of increasing
atomic number and arranges them into groups (vertical columns) and periods
(horizontal rows) according to how the electrons are arranged in each
atom. It turns out that atoms in the same group have similar arrangements
of electrons and similar chemical and physical properties. This can be
very handy in understanding the properties of the elements.
| Copyright owned by the State of Victoria (Department of Education and Early Childhood Development). Used with Permission. |