Molecules have formulas that are set by the capacity of their atoms to form links or bonds with other atoms. The topic From atoms to molecules has many examples of molecules and their formulas. This topic investigates how these formulas are calculated from our knowledge of atoms and their ability to form bonds.
 The ability of an atom to form bonds within a molecule is known as
covalency. Although covalency is very similar to the idea of electrovalency,
the two are often confused. Covalency is used for molecules and electrovalency
is used for ionic solids or salts. Electrovalency is discussed in the
topic Making
salts - the rules of electrovalency.
The ability of an atom to form bonds within a molecule is known as
covalency. Although covalency is very similar to the idea of electrovalency,
the two are often confused. Covalency is used for molecules and electrovalency
is used for ionic solids or salts. Electrovalency is discussed in the
topic Making
salts - the rules of electrovalency.
Covalency
The number of bonds an atom can form within a molecule is known as its
covalency. The simplest atom, hydrogen has the capacity to form one bond;
that is, it can attach itself to one other atom to form a molecule. Oxygen
atoms can form two bonds and nitrogen atoms can form three within a molecule.
Here is a list of common atoms which form molecules with their covalency:
|
1
|
2
|
3
|
4
|
|
hydrogen
|
oxygen
|
nitrogen
|
carbon
|
|
fluorine
|
sulfur
|
phosphorus
|
silicon
|
|
chlorine
|
Matching covalencies
To calculate the formula for a molecule the covalencies of the atoms must
be balanced. Take for instance the molecule methane (CH4) made
from carbon and hydrogen atoms. Carbon with covalency 4 is matched by
4 hydrogen atoms each with covalency 1.

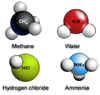
|
Formation of methane. |
Hint:- It is most common for the atom with the highest covalency to form the central atom in the molecule.
Ammonia is made of nitrogen and hydrogen. The central nitrogen atom will combine with three single hydrogen atoms to form NH3.

|
Formation of ammonia. |
Water is made of oxygen and hydrogen. The central oxygen atom will combine with two single hydrogen atoms to form H2O.

|
Covalent formation of water. |
Hydrogen chloride is made of chlorine and hydrogen. The chlorine atom will combine with a single hydrogen atom to form HCl.

|
Formation of hydrogen chloride. |
More combinations
Not all bonds between atoms in a molecule have to be single connections.
Many molecules have double and triple bonds between atoms. Double and
triple bonds in general are stronger than single bonds between the same
atoms. However double or triple bonds are usually much more reactive.
Carbon dioxide (CO2) is a molecule with multiable bonds. Its central carbon atom, covalency 4 has two attached oxygen atoms, each with covalency 2. The covalencies are balanced, ie. 4 = (2x2).

|
Formation of carbon dioxide. |
Try the quiz questions for more examples. Remember the idea is a simple one, although the theory behind it is quite complex. In simple terms, molecules match the covalency of the central atom with those of the surrounding atoms, adding atoms until a balance of covalencies is achieved.
| Copyright owned by the State of Victoria (Department of Education and Early Childhood Development). Used with Permission. |