Corrosion
- two case studies:
aluminium and iron
 |
Aluminium and iron in the form of steel are the
two most widely used metals. Although iron has been in use since
the beginning of the Iron Age (around 1000 BC) this has not always
been the case for aluminium.
|
 |
Although aluminium is very common in the Earth's crust,
the extraction of aluminium from its ore, Bauxite, is difficult and
it has only really been commercially available since the early 1900s.
 |
Both these metals are used in construction and industry
where exposure to water, air and pollution can potentially cause
great damage to the metals. A quick look around the average house
soon shows that if iron, or its alloy steel, is left out in the
wet, it will soon corrode and rust. A piece of aluminium in the
same conditions seems to last quite well, showing little signs of
corrosion. |
 |
Iron
rusts, but aluminium does not
Aluminium really should not be able to be used as it is in high tension
power lines, planes, ships, building and cans to name but a few. Its
relatively high reactivity should make it impossible to use safely.
Aluminium can be made to corrode quickly in air and even burn violently
on heating, but it does not usually react, lasting longer than the less
reactive iron in normal environments.
What is happening?
The chemistry of corrosion
Corrosion, or rusting, occurs when oxygen gas and water react with a
metal to form a new chemical on the surface of the metal called an oxide.
Most metals corrode. For some like sodium, it is such a fast and violent
reaction that the metal burns in contact with water and is soon nothing
but oxide or hydroxides!
At the other end of the spectrum is gold and the other
precious metals which resist corrosion and form only very thin oxide
layers or do not react at all with water and oxygen.
Like most other metals, aluminium and iron react relatively
slowly to form oxides and corrode. The reactions can be represented
by chemical equations.
The two equations for aluminium and iron respectively
are:
Note: This is not the only possible reaction
for iron, but it is a fair representation of the effect of many different
reactions. This process can be speeded up by placing the metals in salt
water.
 Corrosion
experiment Corrosion
experiment
Try a small experiment with steel wool. Place three pieces of steel
wool on a bench top. One is kept dry, one is placed in some fresh water
(do not cover the steel wool with water) and the third placed in salt
water. Watch for a few days. The corrosion will soon become quite evident!
Chemists call corrosion "oxidation". Oxidation literally means
"to combine with oxygen". Corrosion occurs fastest when the
metal is in contact with air, water and salt.
Why does iron rust when aluminium will remain untouched by corrosion?
Permeable vs impervious
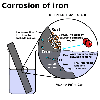 When
it starts to corrode, aluminium forms an oxide layer, which is impervious
to water and oxygen. Iron, on the other hand, forms a permeable oxide
layer which allows both water and oxygen to pass through and keep attacking
the underlying iron. When
it starts to corrode, aluminium forms an oxide layer, which is impervious
to water and oxygen. Iron, on the other hand, forms a permeable oxide
layer which allows both water and oxygen to pass through and keep attacking
the underlying iron.
The impervious aluminium oxide layer protects the aluminium
from further corrosion whereas the rust on the iron flakes off, exposing
more metal iron to attack.
Protecting iron from
rusting
The best way to protect iron or steel is to make an impervious layer
on the metal and protect it the same way as aluminium does naturally.
This is why engineers paint, grease, oil or tar steel to stop water
and oxygen getting to the iron.
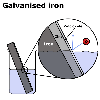 Another
method is to dip the iron in hot liquid zinc. This is called galvanising.
What happens is that the zinc binds to the iron and then corrodes instead
of the iron. An added advantage is that the zinc forms an impervious
oxide layer like aluminium does and this further protects both the iron
and the zinc. Another
method is to dip the iron in hot liquid zinc. This is called galvanising.
What happens is that the zinc binds to the iron and then corrodes instead
of the iron. An added advantage is that the zinc forms an impervious
oxide layer like aluminium does and this further protects both the iron
and the zinc.
Iron and steel can also be protected electrically, by
stopping and reversing the oxidation process. This occurs when lumps
of zinc or magnesium are welded onto the hulls of ships or the piers
of an oil rig. The more reactive zinc or magnesium is corroded before
the iron, thus sacrificing themselves to corrosion instead of the iron.
As a result of this behaviour, zinc or magnesium are known as "sacrificial
anodes"!
|