|
Baking soda and vinegar experiment
This easy to undertake and safe experiment allows students
to observe many of the features of chemical reactions as well as the
three physical states of matter. This experiment clearly distinguishes
a chemical change from physical change.
See the topics Chemical
reactions and Physical
change or chemical change? for more information.
The materials
- Baking soda - Chemical name, sodium bicarbonate with formula NaHCO3.
- Vinegar - A dilute solution of acetic acid in water. Acetic acid
is also known as ethanoic acid, with the formula CH3COOH.
- A beaker or jar.
The chemical reaction
When baking soda is mixed with vinegar, something new is formed. The
mixture quickly foams up with carbon dioxide gas. If enough vinegar
is used, all of the baking soda can be made to react and disappear into
the vinegar solution.
The reaction is:
Sodium bicarbonate and acetic acid reacts to carbon dioxide, water and
sodium acetate.
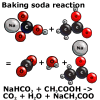
NaHCO3 + CH3COOH  CO2 + H2O + Na CH3COO
CO2 + H2O + Na CH3COO
The physical changes
The solid baking soda was placed in liquid vinegar
producing carbon dioxide gas, which is evident because of the formation
of bubbles in the foaming mixture. Eventually all of the solid dissolved
and reacted producing a new liquid solution.
During the reaction, a solid and liquid have been
chemically reacted to form a gas and a liquid. This experiment can also
be used to explain foams, as liquids or solids containing gas bubbles.
Safety and disposal
Although both reactants are household chemicals and foodstuffs, caution
should be taken not to get splashes in the eyes and clothes should be
protected. The products of the reaction are relatively safe (Remember
- no chemicals should be touched) and can be disposed of by washing
down the sink with plenty of water.
|